Francium By Victor
Francium's symbol is Fr and it's atomic number is 87

Physical Properties
- Very reactive Alkali metal
- MP = 21°C or 294K
- BP = 65°C or 923K
- Number 87
- Normal state is solid
- 223 AMU
What is it like?
- Second most rare of all the elements behind Astatine
- Is red but is hard to tell because there is so little of it
- Very similar to Cs
- Very reactive
Francium
What does this do in the "real world"?
- Only used for research because it is so rare
- Hard to use because it decays very quickly
- Very radioactive
What is Radioactivity?
- In the nuclei of unstable atoms
- It is a very dangerous decay
- 3 types: Alpha decay, Beta decay and Gama decay
- Kills cells in the body
- Can treat cancer by killing cancer cellls
- Since Francium
Bit of History
- Discovered by Marguerite Perey
- Student at the Marie Curie institute
- Discovered in 1939
- Was very patriotic as she named it after France
- Was researching Radioactive decay

In this video you will see Alkali Metals reacting with water. It doesn't actually have Francium in because it is so rare that you can't get a big clump of it. Although the reactions get bigger as you go down the table so you can imagine what would happen with Francium.
Fun Facts
- Currently there isn't enough Francium to weigh
- It is the most unstable natural element
- The last natural element to be discovered
- It had been predicted that there would be a Alkali metal bigger than Cs since 1870
- When found in Uranium it is One Fr atom for a Quintillion U atoms
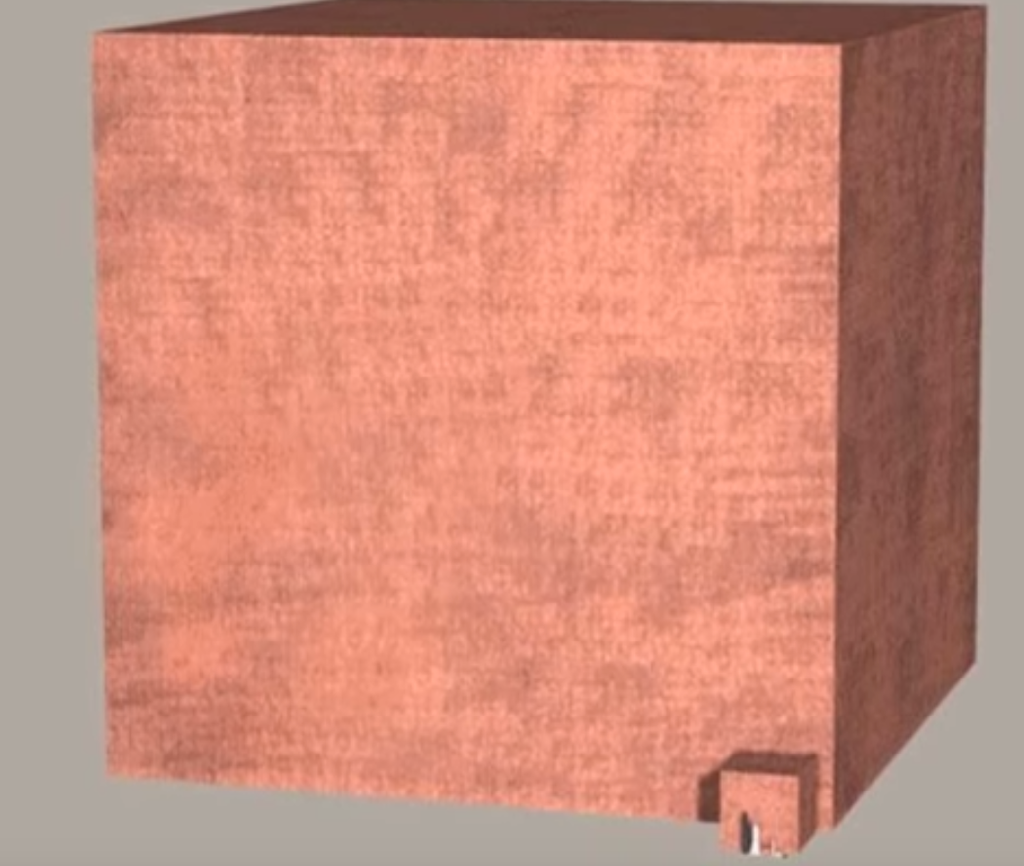
Imagine... This is a quintillion coins then compare it to just one coin. Want to know how big it is? Well those objects in the bottom right are massive buildings such as the Empire State Building and the Washington Monument. Yes, it is that much bigger than those buildings.