Calcium by hadrian reppas
Calcium in Numbers
- Melting point : 942 K
- Atomic number : 20
- Atomic mass : 40.078 amu
- Column number : 2
- Row number : 4
- Symbol : Ca
- Pronunciation : [kal-see-uh m]
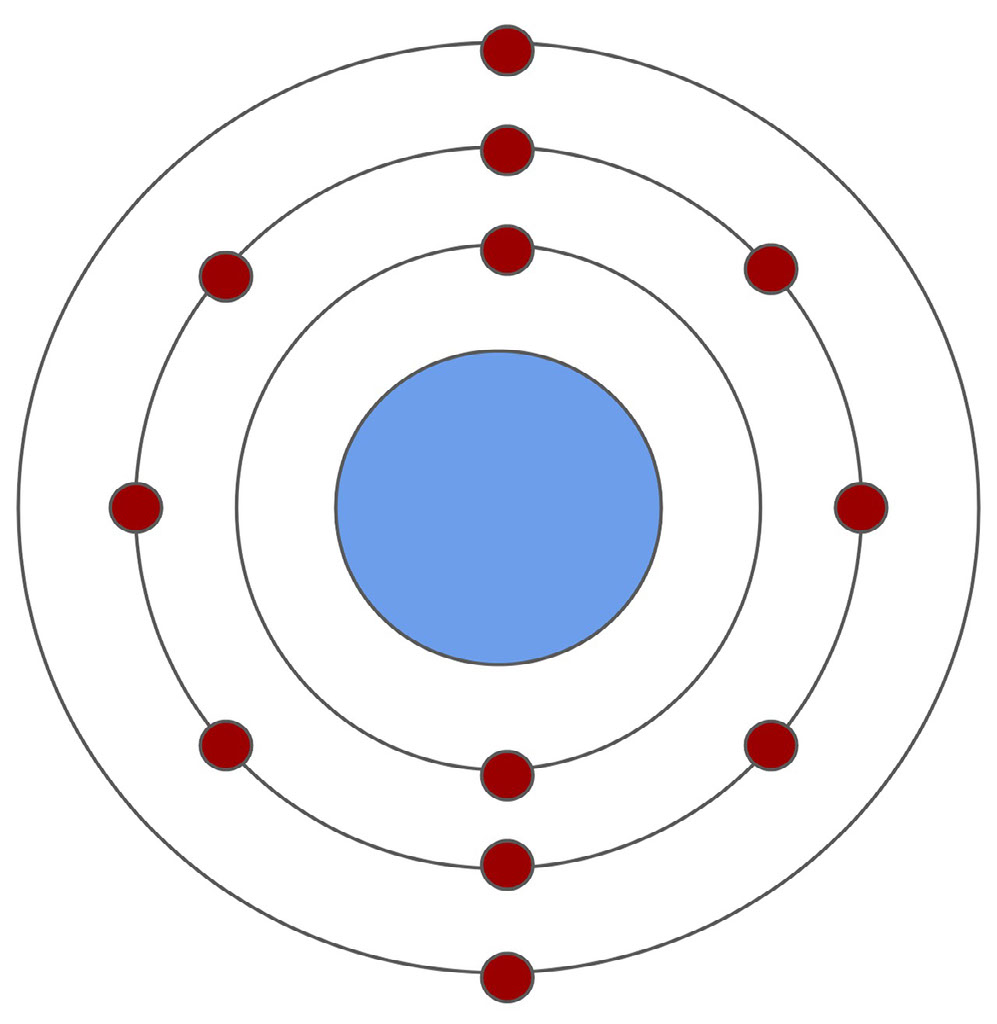
- Two valence electrons

What is Calcium?
- Very common
- 5th most common element in Earth's crust
- Tarnishes in air
- Can't be found in pure form
History of Calcium

- Romans used compounds of calcium
- Sir Humphry Davy purified in 1808
- Named after calx (Latin for limestone)
Uses of Calcium
- Used as reducing agent
- Calcium carbonate (CaCO3) used in concre
- When heated turns into quicklime (CaO)
- calcium sulfate (CaSO4) used in casts
- Used to filter water
Calcium in the Body
- Most abundant mineral in body
- 2% of body is calcium
- 99% in bones and teeth
- 1% for muscles, brain and immunity
- Can lower chances to get cancer