Krypton (Kr) By: Rani Kumar
Physical Properties
- Noble Gas - Non - Metal
- Natural State - Gas.
- Appearance - Colorless and odorless
- Melting Point - 115.79 K.
- Boiling Point - 119.93 K.
- Atomic Number - 36 - Atomic Mass - 83.798
Krypton Glowing In Glass Bottle

History
- Name adapted from greek word hidden - Kryptos
- Discovered by Sir William Ramsay and Morris M. Travers
- Discovered in Britain, May 30th, 1898.
- Discovery made while researching liquified air.

Pictures of the two chemists
Uses
- Fluorescent Lighting
- Flash Lamps - Photography
- Lasers - Krypton Diflouride
- Glowing Signs

Examples
The Isotopes of Krypton
- 6 stable - 30+ unnatural
- Krypton - 86, used in measurement.
- Krypton - 83 used in MRI's.
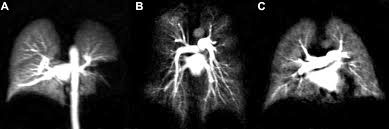
Krypton MRI
Examples
Fun Facts!
- Krypton, one of rarest gases. 0.0001% in our atmosphere.
- Xenon discovered same way, few weeks later.
- Mar's atmosphere - .3 parts per million.

What do you remember?
Krypton was discovered by who?
- A) Sir William Ramsay and J.J. Thomson
- B) Morris M. Travers and Ernest Rutherford
- C) Sir William Ramsay and Morris M. Travers
- D) Niels Bohr and John Dalton.
C) Sir William Ramsay and Morris M. Travers
Krypton can react with fluorine -true or false?
True
What does the Greek word 'kryptos'mean?
- A) Invisible
- B) Hidden
- C) Element
- D) Superman
B) Hidden
How many stable isotopes does krypton have?
- A) 6
- B) 4
- C) 5
- D) 7
A) 6
Dictionary
- Chemical Compunds - Group of chemically bonded elements.
- Fluorine- Element (halogen), highly toxic.
- Isotope - Diffrent variations of one atom.
- Argon - Element (noble gas)
Bibliography




Thanks for watching!
Thanks for watching!